General CHemistry
12.Solids and Modern Materials
Download a PDF worksheet version of this problem set here
Useful Links:
For each type of packing structure listed below, links are available for both Hannah's video walk-through with a model kit or to an interactive external site where you can rotate and manipulate models of these structures.
Download a PDF worksheet version of the answer key here
Study Guide.Crystal Packing
Fill in the following table, which is expanded from your class notes. While you can brute-force memorize your way through this information, it will be much more meaningful if you derive each entry on your own through watching the videos with the model kits or via interactive visualizations of the structures, both linked to the right, or by looking at figures in your textbook.

Hexagonal Close-Packing

The link below directs to the same interactive model external site, but allows you to visualize what some real ionic solid lattices look like. Note that often one ion will sit in the voids of the parent packing pattern.
Study Guide.Polymers
Fill in the table summarizing the kinds of polymers we’ve seen in class. Please note that for many of these columns, especially for applications and examples columns, there are no hard-and-fast answers. There are many good answers, and many, many examples of synthetic polymers. This chart is mostly intended to organize your thoughts.

The coinage metals, Cu, Ag, and Au, all crystalize with a face-centered cubic cell.
a) How many atoms are in a unit cell of one of these metals?
b) What is the coordination number of any given atom?
c) Estimate the length of the cell edge for each metal, given the atomic radii of copper, silver, and gold are 1.45 Å, 1.65 Å, and 1.74 Å, respectively.
d) Calculate the densities of these metals, using the numbers you found in (c).
Additional Polymer Practice Problems
Polypeptides: Why is a peptide bond planar?
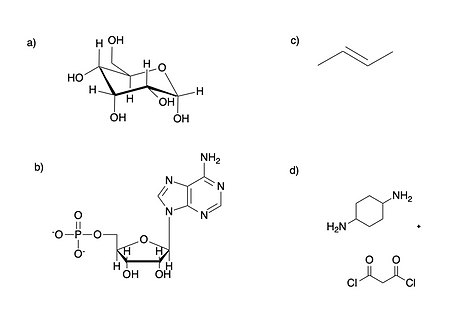
Identify the monomer(s) from the following polymers. What types of polymers are they? What type of reaction was used to synthesize them?

What polymers would the following monomers make?